Each year, billions of vaccine doses are transported across the globe to safeguard populations from infectious diseases. From manufacturing to administration, vaccines must remain within a narrow and strictly controlled temperature range to remain effective. Any deviation—whether from excessive heat or accidental freezing—can compromise the integrity of the vaccine, leading to public health risks, financial loss, and damaged trust in immunization programs.
In this article, we explore the challenges of vaccine transportation, relevant global regulations, and practical solutions to maintain cold chain integrity—focusing especially on how smart monitoring devices can help ensure safety and compliance.
Whether vaccines are shipped locally or internationally, logistics providers must follow strict regulatory frameworks. In the United States, the Food and Drug Administration (FDA) regulates vaccines through its Center for Biologics Evaluation and Research (CBER), requiring manufacturers and transporters to maintain specific conditions throughout the product’s lifecycle.
Globally, organizations like the World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC) publish detailed guidelines and standard operating procedures (SOPs) that specify how vaccines should be stored, handled, and transported. These documents cover everything from temperature parameters to packaging materials and emergency procedures during cold chain failures. Compliance with these guidelines is not only a legal obligation—it is essential to protecting lives.
Different types of vaccines require specific storage conditions:
A single temperature excursion, such as freezing a vaccine that should only be refrigerated, can render it ineffective. Unfortunately, studies have shown that accidental freezing remains a widespread issue, often caused by improper packing or excessive concern over heat exposure.
This underlines the critical need for accurate monitoring tools and validated logistics processes to maintain these temperature thresholds throughout transportation.
Cold chain logistics is complex. Vaccine shipments may pass through multiple transfer points, including warehouses, vehicles, and customs checkpoints. Each hand-off increases the risk of:
While insulation materials and cold packs are commonly used, they are often not enough. Without the ability to measure, record, and act on environmental conditions during transport, cold chain breaches can go undetected until it’s too late.
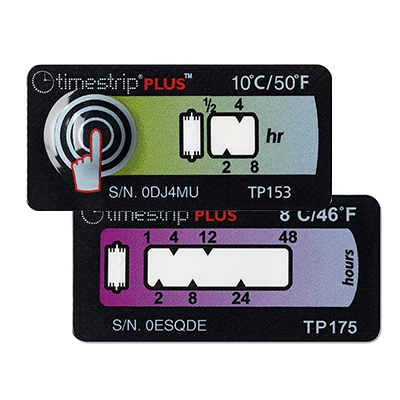
Modern cold chain logistics increasingly relies on smart sensors and indicators to monitor vaccine conditions in real time or on arrival. Key solutions include:
AKS Technology offers a comprehensive portfolio of such monitoring solutions—including the ShockAction and TempAction product lines—designed to support compliance with WHO, CDC, and regional regulatory standards.
Deploying monitoring devices is only one part of the solution. To ensure vaccine safety across the entire cold chain, organizations should also:
By combining best-in-class monitoring with operational discipline, stakeholders can significantly reduce the risks of spoilage and wastage.
Ensuring vaccine safety in transit is a global priority. The cost of failure is not just monetary—it impacts public health and trust. As cold chain logistics grows more complex, smart monitoring technologies offer a powerful way to maintain visibility, accountability, and control across every shipment.
At AKS Technology, we are proud to empower manufacturers, shippers, and health organizations with reliable, certified tools that make a difference. Our mission is simple: help you protect what matters most—one vaccine, one shipment, one life at a time.
By continuing to use the site you agree to our privacy policy Terms and Conditions.